Cushing’s Disease in America 2025
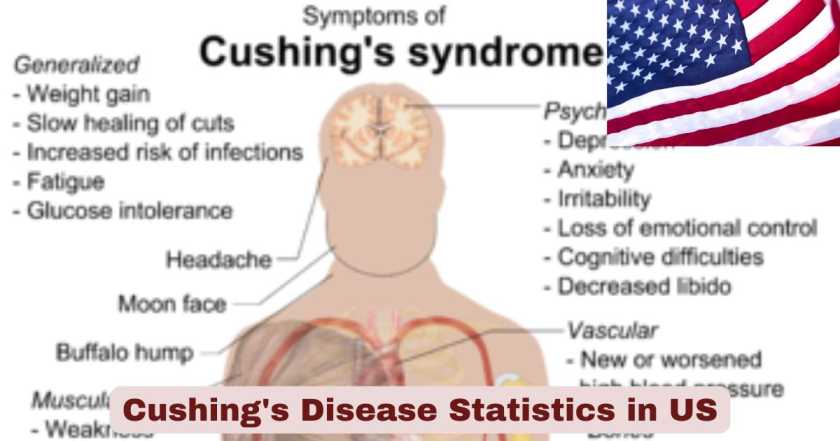
Cushing’s disease continues to represent one of the most challenging endocrine disorders affecting Americans in 2025, characterized by excessive cortisol production due to an ACTH-secreting pituitary adenoma. This rare but serious condition impacts thousands of individuals across the United States, predominantly affecting women between ages 30 and 50 years. The disease carries significant health implications, with patients experiencing elevated risks of cardiovascular complications, metabolic disorders, and substantially reduced quality of life. Current medical research indicates that despite advances in diagnostic techniques and treatment modalities, Cushing’s disease remains underdiagnosed, with many patients experiencing years of symptoms before receiving proper identification and care.
The landscape of Cushing’s disease in America 2025 reflects both progress and persistent challenges. While surgical success rates have improved at specialized centers, achieving 76-80% remission rates, the disease continues to impose substantial burdens on patients, healthcare systems, and society. With an estimated 1.2 to 2.4 cases per million people annually, the rarity of this condition contributes to diagnostic delays averaging 38 months from symptom onset to diagnosis. Mortality rates, though declining since 2000, remain significantly elevated at 2-3 times higher than the general population, underscoring the critical importance of early detection and comprehensive treatment approaches.
Interesting Facts About Cushing’s Disease in the US 2025
| Fact Category | Statistic | Details |
|---|---|---|
| Annual Incidence Rate | 1.2-2.4 cases per million | New diagnoses each year in the United States |
| Gender Distribution | Female-to-Male Ratio 3-5:1 | Women are disproportionately affected |
| Primary Age Group | 30-50 years | Peak diagnosis occurs in fourth decade |
| Average Diagnostic Delay | 38 months | Time from symptom onset to confirmed diagnosis |
| Surgical Success Rate | 76-80% | Initial remission after transsphenoidal surgery |
| Mortality Risk | 2-3 times higher | Compared to general population |
| Recurrence Rate | 10-25% | Patients experiencing disease return after initial remission |
| Healthcare Costs | $26,440 annually | Average per patient in 2025 dollars |
| Cardiovascular Death Rate | 24.7% | Leading cause of mortality among patients |
| Diabetes Prevalence | 30.5% | Patients developing diabetes as comorbidity |
Data sources: National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), PubMed Central medical research databases, and peer-reviewed endocrinology journals (2024-2025)
The statistics presented in this comprehensive table reveal the multifaceted nature of Cushing’s disease and its profound impact on the American population. The incidence rate of 1.2-2.4 cases per million people annually translates to approximately 400-800 new diagnoses each year across the United States, making it a genuinely rare disorder that challenges both patients and healthcare providers. The striking female-to-male ratio of 3-5:1 indicates that women bear a disproportionate burden of this disease, with hormonal and biological factors potentially contributing to this gender disparity. The 38-month average diagnostic delay represents one of the most concerning statistics, as this extended period allows the disease to cause irreversible damage to multiple organ systems, including cardiovascular, metabolic, and skeletal structures.
The surgical success rate of 76-80% demonstrates significant progress in neurosurgical techniques, particularly at experienced pituitary centers where specialized surgeons perform high volumes of transsphenoidal procedures. However, the 10-25% recurrence rate indicates that long-term surveillance remains essential, with many patients requiring additional interventions including repeat surgery, radiation therapy, or medical management. The mortality risk being 2-3 times higher than the general population reflects the severe systemic complications that arise from prolonged cortisol excess, even after successful treatment. Particularly alarming is the 24.7% cardiovascular death rate, which highlights how hypercortisolism damages the heart and blood vessels. The $26,440 annual healthcare cost per patient represents a substantial economic burden, with costs including surgical procedures, medications, diagnostic testing, and management of multiple comorbidities such as the 30.5% of patients who develop diabetes mellitus.
Prevalence and Incidence of Cushing’s Disease in the US 2025
| Measure | 2025 Data |
|---|---|
| Annual Incidence | 1.2-2.4 per million population |
| Estimated New Cases Annually | 400-800 patients |
| Prevalence | 39-40 per million population |
| Total US Cases | Approximately 13,000-14,000 patients |
| Cushing’s Disease as % of All Cushing’s Syndrome | 70-80% |
| Pituitary Microadenomas | 90% |
| Pituitary Macroadenomas | 10% |
Data sources: NIH, NIDDK, European Journal of Endocrinology, Journal of Clinical Endocrinology & Metabolism (2024-2025)
Cushing’s disease prevalence in the US 2025 reflects a relatively stable rare disease burden, with an estimated 39-40 cases per million inhabitants. This translates to approximately 13,000-14,000 Americans currently living with diagnosed Cushing’s disease, though the true number may be higher when considering undiagnosed cases. The annual incidence of 1.2-2.4 per million represents newly diagnosed patients each year, indicating that between 400-800 individuals receive this diagnosis annually across the United States. These figures position Cushing’s disease firmly in the rare disease category, presenting unique challenges for patient advocacy, research funding, and clinical expertise development.
The data reveals that Cushing’s disease accounts for 70-80% of all endogenous Cushing’s syndrome cases, making it the most common cause of pathological hypercortisolism. The distinction between microadenomas (90%) and macroadenomas (10%) is clinically significant, as smaller tumors generally respond better to surgical intervention with higher cure rates and lower complication risks. Microadenomas, defined as pituitary tumors less than 10 millimeters in diameter, present particular diagnostic challenges as they may not be visible on standard MRI imaging in up to 40-50% of cases. This imaging limitation contributes to diagnostic delays and necessitates advanced techniques such as bilateral inferior petrosal sinus sampling (BIPSS) to confirm the pituitary source of ACTH excess. The prevalence data underscores the importance of maintaining specialized pituitary centers with multidisciplinary teams experienced in managing this complex condition.
Demographic Distribution of Cushing’s Disease in the US 2025
| Demographic Factor | Distribution | Percentage/Ratio |
|---|---|---|
| Gender Distribution | Female patients | 75-81% |
| Gender Distribution | Male patients | 19-25% |
| Female-to-Male Ratio | 3-5:1 | Women significantly overrepresented |
| Mean Age at Diagnosis | 39-44 years | Peak incidence fourth decade |
| Age Range Most Affected | 30-50 years | Primary diagnostic window |
| Pediatric Cases | Less than 5% | Rare in children under 18 |
| Ethnicity Data | Limited US-specific data | Appears similar across ethnic groups |
Data sources: MarketScan Commercial Insurance Database, NIH Clinical Studies, Endocrine Practice Journal (2024-2025)
The demographic profile of Cushing’s disease in the US 2025 shows a pronounced gender disparity, with 75-81% of patients being female. This female-to-male ratio of 3-5:1 represents one of the most consistent findings across multiple studies and geographic regions. The biological mechanisms underlying this gender difference remain incompletely understood, but likely involve complex interactions between sex hormones, pituitary cell biology, and potential genetic factors that predispose women to developing ACTH-secreting pituitary adenomas. The mean age at diagnosis of 39-44 years places most patients in their peak working and family-raising years, significantly impacting career trajectories, family planning, and overall life quality.
The 30-50 year age range encompasses the majority of diagnoses, though Cushing’s disease can occur at any age from childhood through late adulthood. Pediatric Cushing’s disease represents less than 5% of all cases but carries unique challenges, including growth retardation, pubertal delays, and psychological impacts during critical developmental periods. In children, the disease often presents with obesity and growth failure as primary symptoms, distinguishing it somewhat from adult presentations. Limited ethnicity-specific data exists for the United States, but available evidence suggests that Cushing’s disease affects individuals across all ethnic groups relatively equally, though access to specialized diagnostic services and treatment may vary by socioeconomic and geographic factors. The demographic patterns emphasize the need for heightened clinical suspicion in women of reproductive age presenting with classic cushingoid features combined with metabolic and cardiovascular complications.
Mortality and Survival Outcomes in Cushing’s Disease in the US 2025
| Mortality Measure | Rate/Risk |
|---|---|
| Overall Mortality Risk | 2-3 times higher than general population |
| Hazard Ratio vs Controls | 1.44-2.1 |
| First Year Mortality Risk | Elevated 3-5 fold |
| Cardiovascular Death | 24.7% of all deaths |
| Infection-Related Death | 12.7-14.4% of all deaths |
| Cerebrovascular Death | 9.4-11.7% of all deaths |
| Thromboembolism Death | 4.2-4.4% of all deaths |
| Mortality in Remission | Still elevated vs general population |
| Mortality with Persistent Disease | 4-fold increase |
| 5-Year Survival (Untreated) | 50% |
Data sources: Swedish Pituitary Register, Danish National Cohort Studies, European Journal of Endocrinology, Journal of Clinical Endocrinology & Metabolism (2024-2025)
The mortality statistics for Cushing’s disease in the US 2025 paint a sobering picture of this condition’s life-threatening nature. Patients face a 2-3 times higher mortality risk compared to age and sex-matched controls from the general population, with the most recent studies reporting hazard ratios of 1.44-2.1. This elevated mortality risk persists even after successful treatment, though it improves significantly compared to patients with active disease. The first year after diagnosis represents a particularly dangerous period, with mortality risk elevated 3-5 fold, primarily due to cardiovascular complications, severe infections, and the stress of surgical intervention.
Cardiovascular disease emerges as the leading cause of death, accounting for 24.7% of all deaths among Cushing’s disease patients. This includes myocardial infarction, heart failure, and sudden cardiac death, reflecting the profound impact of chronic cortisol excess on blood pressure, lipid metabolism, atherosclerosis, and cardiac structure. Infections cause 12.7-14.4% of deaths, with patients experiencing increased susceptibility to bacterial, fungal, and opportunistic infections due to cortisol-induced immunosuppression. Cerebrovascular events, including strokes and aneurysms, account for 9.4-11.7% of deaths, while venous thromboembolism (blood clots) causes 4.2-4.4% of deaths. The 50% five-year survival rate for untreated disease underscores the critical importance of prompt diagnosis and intervention. Even patients achieving biochemical remission maintain elevated mortality risk compared to the general population, emphasizing that Cushing’s disease creates lasting health impacts that extend well beyond cortisol normalization. These statistics highlight the necessity for aggressive cardiovascular risk management, infection prevention strategies, and long-term multidisciplinary follow-up care.
Diagnostic Timeline and Challenges in the US 2025
| Diagnostic Metric | Time/Percentage |
|---|---|
| Average Time to Diagnosis | 38 months (3.2 years) |
| Diagnosis Delay Range | 24-72 months |
| Patients with MRI-Visible Tumor | 41-50% |
| Patients with Negative MRI | 50-59% |
| BIPSS Diagnostic Accuracy | 99% |
| False Positive Rate (Screening) | Variable due to pseudo-Cushing states |
| Patients Seeing Multiple Physicians | Average 4-6 doctors |
| Misdiagnosis Rate | Estimated 30-40% initially |
Data sources: American Academy of Family Physicians, Pituitary Society Consensus Guidelines, Frontiers in Endocrinology (2024-2025)
The diagnostic journey for Cushing’s disease in the US 2025 remains frustratingly prolonged, with patients experiencing an average delay of 38 months from initial symptom onset to confirmed diagnosis. This 3.2-year delay represents a significant quality-of-life burden and allows progressive disease-related damage to accumulate across multiple organ systems. The delay stems from several factors: the insidious onset of symptoms, overlap with common conditions like obesity and diabetes, lack of awareness among primary care physicians, and the complexity of diagnostic testing protocols.
A major diagnostic challenge involves pituitary imaging, with only 41-50% of patients having MRI-visible adenomas at the time of diagnosis. This means that 50-59% of patients have negative or non-diagnostic MRI scans despite having true Cushing’s disease, necessitating additional invasive testing. Bilateral inferior petrosal sinus sampling (BIPSS) has become the gold standard for patients with negative imaging, offering 99% diagnostic accuracy in differentiating pituitary from ectopic sources of ACTH. However, this procedure requires specialized expertise, general anesthesia in some cases, and carries small but real risks of complications. Patients typically consult 4-6 different physicians before receiving the correct diagnosis, often seeing endocrinologists, primary care doctors, gynecologists, cardiologists, and psychiatrists for individual symptoms without recognizing the underlying unified diagnosis. The estimated 30-40% initial misdiagnosis rate reflects how Cushing’s disease symptoms mimic more common conditions, leading to treatments for obesity, depression, diabetes, or hypertension without addressing the root cause. These diagnostic challenges underscore the critical need for increased physician education, improved screening tools, and maintained clinical suspicion in patients presenting with multiple features suggestive of hypercortisolism.
Treatment Outcomes and Success Rates in the US 2025
| Treatment Measure | Success Rate/Outcome |
|---|---|
| First-line Transsphenoidal Surgery Success | 76-80% |
| Microadenoma Surgical Cure Rate | 85-90% |
| Macroadenoma Surgical Cure Rate | 50-65% |
| Recurrence Rate (10 years) | 10-20% |
| Recurrence Rate (Overall) | 10-25% |
| Remission Rate at 1 Year | 80% |
| Remission Rate at 5 Years | 92% |
| Remission Rate at 20 Years | 97% |
| Surgical Mortality | Less than 1% |
| Repeat Surgery Success | 50-70% |
| Radiation Therapy Remission | 50-80% |
| Medical Therapy Response | 40-80% (varies by drug) |
Data sources: Neurosurgery Focus, Registry of Adenomas of the Pituitary and Related Disorders (RAPID), Journal of Clinical Endocrinology & Metabolism (2024-2025)
Treatment success rates for Cushing’s disease in the US 2025 show substantial improvements at experienced centers, with first-line transsphenoidal surgery achieving 76-80% overall remission rates. However, outcomes vary significantly based on tumor characteristics, with microadenomas achieving 85-90% cure rates compared to 50-65% for macroadenomas. This size-dependent success pattern reflects the technical challenges of completely resecting larger tumors that may invade surrounding structures, particularly the cavernous sinus. Surgical expertise matters tremendously, with high-volume neurosurgeons at specialized pituitary centers reporting cure rates approaching 90% even in challenging cases.
The recurrence rate of 10-20% at ten years means that approximately one in five to ten patients who initially achieve remission will experience disease return, necessitating lifelong surveillance through periodic biochemical testing. Long-term data shows remission rates of 80% at one year, 92% at five years, and 97% at twenty years, though these figures include patients who received additional treatments beyond initial surgery. Modern surgical techniques have made the procedure remarkably safe, with mortality rates below 1% at experienced centers. For patients whose initial surgery fails, repeat transsphenoidal surgery offers 50-70% success rates, though with increased risks of pituitary damage and hormone deficiencies. Radiation therapy, including conventional radiotherapy and stereotactic radiosurgery, achieves 50-80% remission rates but typically requires 6-24 months to reach full effectiveness. Medical therapies have expanded significantly, with newer agents like osilodrostat, levoketoconazole, and relacorilant offering response rates of 40-80% depending on the specific medication and disease severity. These therapeutic advances provide important options for patients who cannot undergo surgery or whose surgery was unsuccessful, though medical management typically requires indefinite continuation and carries potential side effects that must be carefully monitored.
Comorbidities and Health Complications in the US 2025
| Comorbidity | Prevalence in Patients |
|---|---|
| Hypertension | 55-75% |
| Diabetes Mellitus | 30.5-40% |
| Obesity (BMI >30) | 60-65% |
| Osteoporosis | 50-80% |
| Depression | 50-70% |
| Anxiety Disorders | 40-60% |
| Cardiovascular Disease | 8-15% |
| Infections (Serious) | 21% |
| Kidney Stones | 5.5-10% |
| Vertebral Fractures | 10-40% |
| Muscle Weakness/Myopathy | 60-80% |
| Sleep Disorders | 70-80% |
Data sources: MarketScan Commercial Insurance Databases, Endocrine Practice, Pituitary Journal (2024-2025)
The comorbidity burden in Cushing’s disease patients in the US 2025 is extensive and multisystemic, with hypertension affecting 55-75% of patients. This elevated blood pressure stems from multiple mechanisms including increased sodium retention, enhanced vascular reactivity, and elevated production of other mineralocorticoids. Diabetes mellitus develops in 30.5-40% of patients as cortisol induces insulin resistance and impairs pancreatic beta-cell function. Obesity, particularly central adiposity, affects 60-65% of patients, with characteristic fat deposition in the face (moon facies), upper back (buffalo hump), and abdomen.
Bone health represents a major concern, with osteoporosis occurring in 50-80% of patients and vertebral compression fractures in 10-40%. Cortisol directly inhibits bone formation while increasing bone resorption, leading to rapid bone loss that may not fully reverse even after successful treatment. Psychiatric manifestations are nearly universal, with depression affecting 50-70% and anxiety disorders impacting 40-60% of patients. These psychological symptoms significantly impair quality of life and may persist long after biochemical cure. Cardiovascular disease affects 8-15% of patients at diagnosis, including coronary artery disease, heart failure, and left ventricular hypertrophy. Serious infections occur in 21% of patients due to cortisol-induced immunosuppression, with risks for bacterial, fungal, and opportunistic pathogens. Muscle weakness and myopathy affect 60-80% of patients, causing difficulty climbing stairs, rising from chairs, and performing daily activities. Sleep disorders, including insomnia and sleep apnea, impact 70-80% of patients, further compromising quality of life and metabolic health. This extensive comorbidity profile necessitates comprehensive multidisciplinary care extending well beyond endocrine management to address cardiovascular, bone, mental health, and infectious disease risks.
Healthcare Costs and Economic Burden in the US 2025
| Cost Category | Annual Amount |
|---|---|
| Total Annual Healthcare Costs per Patient | $26,440 |
| Costs vs Non-Functioning Pituitary Adenomas | $13,708 |
| Costs vs General Population | $5,954 |
| Inpatient Hospitalization Rate | 19.3% |
| Emergency Department Visit Rate | 25.4% |
| Outpatient Visit Costs | Significantly elevated |
| Pharmacy Costs | Significantly elevated |
| Costs for Patients Not in Remission | Nearly double remission costs |
| Patient Out-of-Pocket Annual Costs | $9,000-11,000 |
| Disability/Lost Productivity | 30-50% workforce reduction |
| Treatment Market Value (2024) | $1.2 billion |
| Projected Market (2033) | $1.9 billion |
Data sources: MarketScan Insurance Databases, Endocrine Practice, Partnership for Health Analytic Research (2024-2025)
The economic burden of Cushing’s disease in the US 2025 is substantial, with total annual healthcare costs averaging $26,440 per patient. This figure is nearly double the $13,708 spent on patients with non-functioning pituitary adenomas and more than four times the $5,954 spent on matched general population controls. These elevated costs reflect the complex, multisystem nature of the disease requiring frequent specialist visits, advanced imaging, hormonal testing, surgical interventions, medications, and management of multiple comorbidities. Hospitalization rates reach 19.3% annually, significantly higher than the 5.6% rate in the general population, driven by complications including cardiovascular events, infections, metabolic crises, and surgical admissions.
Emergency department utilization occurs in 25.4% of patients annually, compared to 14.3% in general population controls, reflecting the acute complications that can arise from hypercortisolism. Outpatient and pharmacy costs are both significantly elevated, with patients requiring multiple specialty visits to endocrinologists, neurosurgeons, cardiologists, and other specialists, plus expensive medications for hypercortisolism and associated comorbidities. Patients not achieving remission experience costs nearly double those in remission, highlighting the cost-effectiveness of successful surgical treatment. Individual patient out-of-pocket expenses range from $9,000-11,000 annually, including copayments, deductibles, medications, transportation, and uncovered services. The indirect costs through disability and lost productivity may actually exceed direct medical costs, with 30-50% of patients experiencing significant workforce reduction or disability. The Cushing’s disease treatment market was valued at $1.2 billion in 2024 and projects to reach $1.9 billion by 2033, reflecting ongoing pharmaceutical development and growing recognition of the disease. These economic figures emphasize both the individual financial burden on patients and families and the broader societal costs of this rare but impactful condition.
Quality of Life Impact in the US 2025
| Quality of Life Measure | Impact Level |
|---|---|
| Overall QoL Reduction | Significantly impaired vs healthy controls |
| Physical Function | Substantially reduced |
| Mental Health/Emotional | Substantially reduced |
| Social Function | Substantially reduced |
| Fatigue Levels | Severe in 70-80% |
| Depression Scores | Elevated in 50-70% |
| Anxiety Scores | Elevated in 40-60% |
| Cognitive Impairment | Memory and executive function deficits |
| Sexual Function | Significantly impaired |
| Employment Impact | 30-50% reduction in work capacity |
| QoL After Remission | Improved but not normalized |
| Persistent QoL Deficits | Last 10+ years post-treatment |
Data sources: CushingQoL Questionnaire Studies, Hospital Anxiety and Depression Scale, Nottingham Health Profile, Pituitary Journal (2024-2025)
The quality of life impact of Cushing’s disease in the US 2025 is profound and multidimensional, with patients experiencing significantly reduced wellbeing across physical, mental, emotional, and social domains compared to healthy controls. Even after successful treatment and biochemical remission, many aspects of quality of life remain impaired, sometimes permanently. Physical function is substantially compromised, with patients reporting difficulties with routine activities like climbing stairs, carrying groceries, and performing household tasks due to muscle weakness, fatigue, and bone/joint pain.
Severe fatigue affects 70-80% of patients, representing one of the most disabling symptoms that persists long after cortisol normalization. This fatigue is distinct from normal tiredness, involving overwhelming exhaustion that doesn’t improve with rest. Depression impacts 50-70% of patients with scores on validated instruments significantly elevated compared to population norms. The depression associated with Cushing’s disease involves both direct biochemical effects of cortisol on brain function and reactive elements related to physical changes, social stigma, and chronic illness burden. Anxiety disorders affect 40-60% of patients, including generalized anxiety, social anxiety, and panic symptoms. Cognitive impairment is nearly universal during active disease, with deficits in memory, concentration, executive function, and processing speed that may improve but often don’t fully normalize after treatment.
Sexual function is significantly impaired through multiple mechanisms including hormonal imbalances, mood disturbances, physical changes affecting body image, and medication side effects. The employment impact is severe, with 30-50% of patients experiencing substantial work capacity reduction, including disability leave, part-time status reduction, or early retirement. Studies using disease-specific instruments like the CushingQoL questionnaire consistently demonstrate that while quality of life improves following successful treatment, it typically does not return to normal levels. Persistent quality of life deficits can last 10 or more years post-treatment, with patients reporting ongoing issues with fatigue, mood, cognition, and physical limitations. The presence of hypopituitarism (damage to other pituitary hormones) following surgery particularly predicts worse long-term quality of life outcomes. These findings emphasize that Cushing’s disease creates lasting impacts extending far beyond cortisol normalization, requiring comprehensive long-term supportive care addressing physical rehabilitation, mental health, vocational support, and social reintegration.
Pediatric Cushing’s Disease Statistics in the US 2025
| Pediatric Measure | Rate/Outcome |
|---|---|
| Pediatric Cases | Less than 5% of all CD |
| Mean Age at Surgery | 13.7 years |
| Age Range | 4-21 years |
| Surgical Remission Rate | 82-96% |
| MRI Positive Rate | 50% |
| BIPSS Diagnostic Accuracy | 99% |
| Mortality Rate | 2.5% |
| Primary Cause of Death | Sepsis (75%) |
| Growth Impairment | Common presenting feature |
| Obesity | Present in majority |
| Pubertal Delay | Common |
| Long-term QoL Impact | Persistent deficits in some |
Data sources: National Institutes of Health Pediatric Studies, European Journal of Pediatric Endocrinology, Neurosurgery (2024-2025)
Pediatric Cushing’s disease represents less than 5% of all cases but carries unique diagnostic and treatment challenges in the US 2025. Children typically present at a mean age of 13.7 years, though cases occur throughout childhood and adolescence from ages 4-21 years. Pediatric presentations differ somewhat from adults, with growth failure and obesity being predominant features. The slowing or cessation of linear growth despite increasing weight is a particularly concerning sign that should prompt endocrine evaluation.
Surgical outcomes in children are generally favorable, with remission rates of 82-96% at specialized pediatric pituitary centers. However, only 50% of pediatric patients have MRI-visible adenomas, necessitating bilateral inferior petrosal sinus sampling (BIPSS) in many cases, which achieves 99% diagnostic accuracy. The procedure requires general anesthesia in young children, adding complexity. While rare, pediatric mortality of 2.5% has been documented at specialized centers, with 75% of deaths attributable to sepsis (severe infections), highlighting the critical importance of infection prevention and vigilant monitoring. Growth impairment is often dramatic, with children falling off their growth curves while simultaneously gaining excessive weight in characteristic truncal and facial distribution. Pubertal delay is common, with adolescents experiencing arrested sexual development that may resume after successful treatment. Long-term quality of life studies show that some children who are treated for Cushing’s disease continue to experience physical and psychosocial difficulties into adulthood, though outcomes appear better than adults treated for disease acquired in adulthood. Early diagnosis and treatment are particularly critical in children to minimize long-term sequelae affecting growth, development, bone health, and psychological wellbeing.
Current Treatment Landscape and Emerging Therapies in the US 2025
| Treatment Category | Options and Efficacy |
|---|---|
| First-line Treatment | Transsphenoidal surgery |
| FDA-Approved Medical Therapies | Pasireotide, Osilodrostat, Levoketoconazole, Mifepristone |
| Pasireotide Response Rate | 15-30% |
| Osilodrostat Response Rate | 53-86% |
| Mifepristone Response Rate | Variable (glucocorticoid receptor blockade) |
| Bilateral Adrenalectomy | 100% biochemical cure |
| Radiation Therapy | 50-80% remission (over 2-5 years) |
| Stereotactic Radiosurgery | 54-83% remission |
| Emerging Therapies in Trials | Relacorilant, Novel targeted agents |
| Combination Medical Therapy | 88% response in small studies |
Data sources: FDA Approvals, Clinical Trials.gov, New England Journal of Medicine, Lancet Diabetes & Endocrinology (2024-2025)
The treatment landscape for Cushing’s disease in the US 2025 has expanded significantly with multiple FDA-approved medications complementing surgical approaches. Transsphenoidal surgery remains the first-line treatment for virtually all patients with confirmed Cushing’s disease, offering the best chance for cure with immediate effect. However, for patients who are not surgical candidates, have persistent disease after surgery, or experience recurrence, several medical therapies now provide important options.
Pasireotide (Signifor), a somatostatin analog, was the first medication approved specifically for Cushing’s disease, achieving 15-30% response rates as monotherapy. Osilodrostat (Isturisa), an 11β-hydroxylase inhibitor, has demonstrated superior efficacy with 53-86% response rates in normalizing cortisol levels, making it an increasingly important option. Levoketoconazole (Recorlev), an improved form of ketoconazole, offers cortisol normalization in approximately 40-50% of patients with better tolerability than the older racemic mixture. Mifepristone (Korlym), a glucocorticoid receptor antagonist, provides unique mechanism blocking cortisol action rather than reducing production, particularly valuable for patients with diabetes or glucose intolerance. Bilateral adrenalectomy represents a definitive surgical option achieving 100% biochemical cure by removing cortisol-producing glands, though creating lifelong adrenal insufficiency requiring replacement therapy and carrying risk of Nelson’s syndrome (aggressive ACTH tumor growth) in 8-29% of patients.
Radiation therapy options include conventional fractionated radiotherapy and stereotactic radiosurgery (Gamma Knife or CyberKnife), with remission rates of 50-80% and 54-83% respectively, though effects develop gradually over 2-5 years. Emerging therapies show promise, with relacorilant, a selective glucocorticoid receptor modulator, demonstrating encouraging results in clinical trials with potentially fewer side effects than mifepristone. Combination medical therapy represents an evolving strategy, with small studies showing 88% response rates when combining medications with different mechanisms, such as osilodrostat plus cabergoline. This expanding therapeutic armamentarium provides clinicians with multiple options for managing this complex disease, though individual patient factors, comorbidities, disease severity, and preference all influence optimal treatment selection.
Diagnostic Testing and Biomarkers in the US 2025
| Diagnostic Test | Sensitivity/Specificity |
|---|---|
| 24-hour Urinary Free Cortisol | Sensitivity 90%, Specificity 68% |
| Late-night Salivary Cortisol | Sensitivity 92-100%, Specificity 93-96% |
| Low-dose Dexamethasone Suppression | Sensitivity 95-100%, Specificity variable |
| BIPSS for Localization | Sensitivity 96%, Specificity 95% |
| BIPSS with CRH Stimulation | Sensitivity 99%, Specificity 100% |
| Pituitary MRI Detection Rate | 41-50% |
| Plasma ACTH Levels | Essential for differential diagnosis |
| High-dose Dexamethasone Test | Less commonly used in 2025 |
| CRH Stimulation Test | Adjunct to BIPSS |
Data sources: Endocrine Society Clinical Practice Guidelines, Journal of Clinical Endocrinology & Metabolism, Frontiers in Endocrinology (2024-2025)
Diagnostic testing for Cushing’s disease in the US 2025 employs multiple biochemical assessments, each with distinct advantages and limitations. The 24-hour urinary free cortisol (UFC) measurement demonstrates 90% sensitivity but only 68% specificity, meaning false positives occur frequently with physiological stress, depression, obesity, or other conditions. Multiple collections improve accuracy. Late-night salivary cortisol has emerged as particularly valuable, offering 92-100% sensitivity and 93-96% specificity while being convenient and non-invasive, measuring cortisol at its nadir when healthy individuals have very low levels.
The overnight low-dose dexamethasone suppression test achieves 95-100% sensitivity for detecting Cushing’s syndrome, though specificity varies as some patients fail to suppress normally despite not having true disease. Once hypercortisolism is confirmed, distinguishing Cushing’s disease (pituitary source) from other causes requires plasma ACTH measurement and often bilateral inferior petrosal sinus sampling (BIPSS). BIPSS achieves remarkable accuracy with 96% sensitivity, 95% specificity, improving to 99% sensitivity and 100% specificity when combined with CRH or desmopressin stimulation. This invasive procedure involves catheterizing veins draining the pituitary to measure ACTH gradients, definitively establishing pituitary versus ectopic sources. Pituitary MRI visualization of adenomas occurs in only 41-50% of cases, necessitating biochemical confirmation via BIPSS when imaging is negative. The high-dose dexamethasone suppression test, once commonly used, has fallen out of favor as it’s less reliable than BIPSS. This comprehensive diagnostic approach, while complex, achieves high accuracy essential for directing appropriate therapy in this challenging condition where misdiagnosis could lead to unnecessary or insufficient treatment.
Recurrence and Long-term Surveillance in the US 2025
| Surveillance Measure | Rate/Recommendation |
|---|---|
| Overall Recurrence Rate | 10-25% |
| Early Recurrence (within 5 years) | 5-10% |
| Late Recurrence (after 5 years) | 5-15% |
| Time to Recurrence | Average 48-60 months |
| Recommended Follow-up Duration | Lifelong |
| Initial Post-op Testing | Daily cortisol for 2 weeks |
| First Year Testing Frequency | Every 3 months |
| Long-term Testing Frequency | Annually if stable |
| MRI Surveillance | Annually for 5 years, then as indicated |
| Predictors of Recurrence | Incomplete resection, higher pre-op cortisol |
Data sources: Pituitary Society Guidelines, Journal of Neurosurgery, Endocrine Practice (2024-2025)
Recurrence of Cushing’s disease affects 10-25% of patients in the US 2025 who initially achieve surgical remission, making lifelong surveillance essential. Early recurrence within the first five years occurs in 5-10% of cases, while late recurrence after five years affects an additional 5-15%, with some recurrences documented more than decade after initial successful surgery. The average time to recurrence is 48-60 months, though wide variability exists with some patients experiencing very early recurrence within months and others remaining disease-free for decades before recurrence.
Post-operative surveillance protocols in 2025 emphasize intensive early monitoring with daily morning cortisol measurements for the first 2 weeks after surgery, as post-operative hypocortisolism (cortisol deficiency requiring replacement) is actually a favorable prognostic sign suggesting complete adenoma removal. Testing frequency follows a 3-month interval for the first year, then transitions to annual biochemical assessment including 24-hour urinary free cortisol, late-night salivary cortisol, and sometimes low-dose dexamethasone suppression testing. Pituitary MRI is typically performed annually for five years post-surgery, then less frequently if biochemical remission is sustained, though any biochemical evidence of recurrence triggers immediate imaging. Predictors of higher recurrence risk include evidence of incomplete tumor resection at initial surgery, persistently elevated immediate post-operative cortisol levels, very high pre-operative cortisol levels, macroadenomas, and invasive tumor characteristics. Patients and providers must maintain vigilance throughout the patient’s life, as the risk never completely disappears. This surveillance burden adds to the psychological and practical challenges of living with Cushing’s disease, even after successful treatment, as patients never receive definitive clearance that their disease will not return.
Associated Conditions and Syndrome Variants in the US 2025
| Associated Condition | Prevalence/Details |
|---|---|
| Cushing’s Disease vs Cushing’s Syndrome | CD is 70-80% of endogenous CS |
| Ectopic ACTH Syndrome | 10-15% of ACTH-dependent CS |
| Adrenal Cushing’s Syndrome | 15-20% of endogenous CS |
| Iatrogenic Cushing’s Syndrome | Most common overall form |
| Cyclic Cushing’s Syndrome | 15% of CD patients |
| MEN1-Associated CD | 2-3% of CD cases |
| Carney Complex CD | Rare, <1% |
| McCune-Albright Syndrome | Very rare cause |
| Pseudo-Cushing’s States | Depression, alcoholism, obesity |
Data sources: NIH Genetic and Rare Diseases Information Center, European Journal of Endocrinology, Orphanet Journal of Rare Diseases (2024-2025)
Understanding the relationship between Cushing’s disease and related conditions is essential in the US 2025. Cushing’s disease specifically refers to pituitary-dependent ACTH excess and represents 70-80% of endogenous (internally produced) Cushing’s syndrome cases. Ectopic ACTH syndrome, where ACTH production originates from non-pituitary tumors (most commonly lung carcinoids or small cell lung cancer), accounts for 10-15% of ACTH-dependent cases and requires distinct treatment approaches targeting the underlying malignancy. Adrenal Cushing’s syndrome, caused by cortisol-secreting adrenal tumors independent of ACTH, represents 15-20% of endogenous cases.
Iatrogenic Cushing’s syndrome, resulting from prescribed glucocorticoid medications for inflammatory or autoimmune conditions, is actually the most common form of Cushing’s syndrome overall, far exceeding endogenous causes, though it is excluded from Cushing’s disease statistics. Cyclic Cushing’s syndrome presents unique diagnostic challenges, occurring in approximately 15% of Cushing’s disease patients where cortisol production fluctuates with periods of normal and elevated levels, requiring repeated testing to capture hypercortisolism episodes. Multiple Endocrine Neoplasia Type 1 (MEN1) syndrome, a genetic condition causing multiple endocrine tumors, is associated with Cushing’s disease in 2-3% of cases and requires genetic counseling and family screening. Carney Complex and McCune-Albright syndrome are extremely rare genetic conditions that can present with Cushing’s syndrome through distinct mechanisms. Pseudo-Cushing’s states, particularly depression, chronic alcoholism, and severe obesity, can produce biochemical test abnormalities mimicking true Cushing’s syndrome, contributing to diagnostic complexity and false-positive results requiring expert interpretation to distinguish from authentic disease. This spectrum of related conditions emphasizes why expert endocrine evaluation is crucial for accurate diagnosis and appropriate treatment selection.
Gender-Specific Considerations in the US 2025
| Gender Consideration | Impact/Details |
|---|---|
| Female Predominance | 75-81% of all cases |
| Reproductive Age Women | Highest risk group |
| Menstrual Irregularities | 80-90% of female patients |
| Fertility Impact | Significantly reduced |
| Pregnancy Complications | Increased risk of multiple complications |
| Hirsutism/Virilization | 50-80% of female patients |
| Male Sexual Dysfunction | Common in male patients |
| Testosterone Reduction in Men | Frequent finding |
| Pregnancy-Associated CD | Rare but dangerous |
| Postmenopausal Diagnosis | Later diagnosis common |
Data sources: Journal of Clinical Endocrinology & Metabolism, European Journal of Endocrinology, Fertility and Sterility (2024-2025)
Gender-specific aspects of Cushing’s disease in the US 2025 extend beyond the basic female predominance of 75-81% to encompass distinct clinical presentations and complications. Reproductive-age women (ages 25-45) represent the highest risk demographic, with hormonal factors potentially contributing to adenoma development. Menstrual irregularities affect 80-90% of premenopausal women, including oligomenorrhea (infrequent periods), amenorrhea (absent periods), and irregular cycles, often prompting gynecologic evaluation before the underlying Cushing’s disease is recognized.
Fertility is significantly impaired in women with active Cushing’s disease, with many experiencing anovulation (lack of ovulation) and difficulty conceiving. For women who do become pregnant with active disease, pregnancy complications are substantially elevated, including gestational diabetes, preeclampsia, preterm delivery, fetal growth restriction, and increased maternal and fetal mortality risk. Hirsutism (excess facial and body hair) and virilization (male-pattern features) affect 50-80% of women with Cushing’s disease due to elevated adrenal androgens, causing significant psychological distress and social stigma. Male patients experience their own set of reproductive complications, including erectile dysfunction, reduced libido, and decreased testosterone levels, contributing to quality of life impairment. Pregnancy-associated Cushing’s disease is rare but particularly dangerous, with maternal mortality rates historically reaching 2-10% though improving with modern management. Diagnosis during pregnancy is complicated by normal pregnancy-related increases in cortisol levels. Postmenopausal women may experience later diagnosis as symptoms like weight gain, mood changes, and fatigue are sometimes attributed to normal aging or menopause rather than investigated as potential Cushing’s disease. These gender-specific considerations emphasize the need for heightened clinical awareness in appropriate populations and specialized management approaches addressing reproductive health concerns.
Regional and Healthcare Access Disparities in the US 2025
| Access Factor | Impact |
|---|---|
| Specialized Pituitary Centers | Concentrated in major academic medical centers |
| Rural Access Challenges | Significant barriers to specialized care |
| Average Travel Distance for Surgery | Often exceeds 100 miles |
| Diagnosis Delay in Underserved Areas | Extended beyond national average |
| Insurance Coverage Variations | Significant impact on treatment access |
| Medicaid/Uninsured Patients | Face substantial barriers |
| Telemedicine Utilization | Growing for follow-up care |
| Clinical Trial Participation | Limited outside major centers |
| Genetic Testing Access | Variable by region/insurance |
| Multidisciplinary Team Access | Limited in smaller centers |
Data sources: American Association of Neurological Surgeons, Health Resources and Services Administration, Healthcare Cost and Utilization Project (2024-2025)
Healthcare access disparities for Cushing’s disease in the US 2025 create significant inequalities in diagnosis speed, treatment quality, and outcomes. Specialized pituitary centers with high-volume neurosurgeons experienced in transsphenoidal surgery are concentrated at major academic medical centers in urban areas, leaving rural and underserved populations with limited local access. Patients often must travel 100 miles or more for surgical treatment, creating financial burdens, logistical challenges, and delays in care. Rural patients experience extended diagnostic delays beyond the already concerning 38-month national average, as local healthcare providers may have less familiarity with rare endocrine disorders and limited access to specialized testing like BIPSS.
Insurance coverage dramatically impacts treatment access, with some plans limiting access to out-of-network specialized centers or requiring extensive prior authorizations for expensive medications like osilodrostat or levoketoconazole. Medicaid and uninsured patients face substantial barriers to accessing optimal care, though federal law mandates emergency treatment and many academic centers provide charity care programs. Telemedicine has expanded significantly post-COVID-19 pandemic, enabling remote follow-up consultations with pituitary specialists, though initial diagnosis and surgical treatment still require in-person evaluation. Clinical trial participation, offering access to emerging therapies, is predominantly available at major research centers, limiting opportunities for patients in other regions. Genetic testing for conditions like MEN1 has variable insurance coverage and may be financially prohibitive for some families. The multidisciplinary team approach recommended for comprehensive Cushing’s disease management (involving endocrinologists, neurosurgeons, radiation oncologists, psychiatrists, physical therapists) is readily available at specialized centers but difficult to coordinate in smaller community hospitals. These disparities contribute to outcome inequalities, with patients treated at high-volume specialized centers achieving better remission rates and fewer complications than those treated at low-volume centers, raising questions about equity and optimal healthcare delivery models for rare diseases.
Emerging Research and Future Directions in the US 2025
| Research Area | Development Stage |
|---|---|
| Novel Medical Therapies | Multiple agents in clinical trials |
| Targeted Molecular Therapies | Preclinical and early clinical studies |
| Improved Imaging Techniques | Ongoing development |
| Genetic Risk Factors | Active investigation |
| Biomarkers for Diagnosis | Research stage |
| Liquid Biopsy Techniques | Exploratory |
| Artificial Intelligence Diagnostics | Early implementation |
| Gene Therapy | Conceptual stage |
| Personalized Medicine Approaches | Emerging |
| Quality of Life Interventions | Ongoing trials |
Data sources: ClinicalTrials.gov, Nature Endocrinology, Lancet Diabetes & Endocrinology, Pituitary Society Research Updates (2024-2025)
Emerging research for Cushing’s disease in the US 2025 encompasses multiple exciting directions promising improved diagnosis, treatment, and outcomes. Novel medical therapies under investigation include new steroidogenesis inhibitors with improved efficacy and tolerability profiles, next-generation selective glucocorticoid receptor modulators, and combination therapy regimens optimizing multiple mechanisms simultaneously. Targeted molecular therapies aim to address specific genetic and molecular abnormalities driving corticotroph adenoma growth, with research identifying potential targets including USP8 mutations (found in approximately 35-60% of corticotroph adenomas), epidermal growth factor receptor (EGFR) pathway components, and cell cycle regulators.
Advanced imaging techniques including 7-Tesla MRI, specialized pituitary protocols, and functional imaging methods aim to improve the currently disappointing 41-50% detection rate of pituitary microadenomas on conventional imaging. Genetic research investigates both germline predisposition factors and somatic tumor mutations, with potential implications for risk stratification, family screening, and targeted therapies. Novel biomarkers under development seek to improve diagnostic accuracy, predict treatment response, and identify recurrence earlier than current biochemical methods. Liquid biopsy techniques analyzing circulating tumor DNA or extracellular vesicles represent cutting-edge approaches potentially enabling minimally invasive tumor characterization. Artificial intelligence and machine learning algorithms are being developed to assist with radiological tumor detection, predict surgical outcomes, and identify subtle patterns in biochemical testing that human interpretation might miss. While gene therapy remains conceptual for Cushing’s disease, advances in other pituitary conditions suggest future possibilities. Personalized medicine approaches aim to match individual patients with optimal treatments based on genetic profiles, tumor characteristics, and comorbidity patterns. Research into quality of life interventions includes cognitive rehabilitation programs, targeted physical therapy protocols, and psychological interventions specifically designed for Cushing’s disease patients addressing persistent post-remission symptoms. These research directions offer hope for continued improvements in this challenging condition, though translation from research to clinical practice typically requires years of rigorous testing and regulatory approval.
Disclaimer: This research report is compiled from publicly available sources. While reasonable efforts have been made to ensure accuracy, no representation or warranty, express or implied, is given as to the completeness or reliability of the information. We accept no liability for any errors, omissions, losses, or damages of any kind arising from the use of this report.